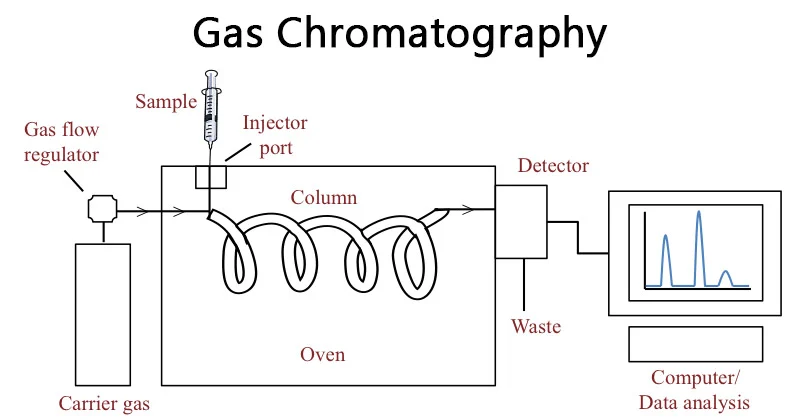

The quality of analysis depends more on the quality of the operator than that of the instrument, so this course will help laboratory staff to understand meaning and principals of gas chromatographic with method development and validation for several application.
The course is of interest for any person working in any analytical laboratory. Laboratory staff, Chemists, supervisors and technicians.
This interactive Training will be highly interactive, with opportunities to advance your opinions and ideas and will include;
Day 1:
Day 2:
Day 3:
Day 4:
Day 5:
CDGA attendance certificate will be issued to all attendees completing minimum of 85% of the total course duration.
| Code | Date | Venue | Fees | Register |
|---|---|---|---|---|
| LAB164-02 | 07-06-2026 | Manama | USD 5450 | |
| LAB164-03 | 06-09-2026 | Cairo | USD 5450 | |
| LAB164-04 | 13-12-2026 | Dubai | USD 5450 |
Providing services with a high quality that are satisfying the requirements
Appling the specifications and legalizations to ensure the quality of service.
Best utilization of resources for continually improving the business activities.
CDGA keen to selects highly technical instructors based on professional field experience
Since CDGA was established, it considered a training partner for world class oil & gas institution
3012, Block 3, 30 Euro Business Park, Little Island, Co. Cork, T45 V220, Ireland
Mon to Fri 09:00 AM to 06:00 PM
Contact Us anytime!
Request Info